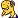
Measuring Acid Rain
The pH (not PH) scale is used to measure the acidity or alkalinity of an aqueous solution and is determined by the hydrogen ion content (H+). This scale was invented by a Danish scientist called Sorenson in 1909. The pH scale ranges from 0, which is strongly acid, to 14 which is strongly alkaline, the scale point 7 being neutral. Examples of solutions with differing pH values include car battery acid (pH 1), lemon juice (pH 2), beer (pH 4), natural rain (pH 5-6), milk (pH 6), washing-up liquid (pH 7), seawater (pH 8), milk of magnesia (pH 10) and ammonia (pH 12),
The pH scale is logarithmic rather than linear, and so there is a ten fold increase in acidity with each pH unit, such that rainfall with pH 5 is ten times more acidic than pH 6, rainfall with pH 4 is 100 times more acidic than pH 6 and rainfall with pH 3 is 1000 times more acidic than pH 6.
Rainfall acidity is measured in pH units. Normal or unpolluted rainfall has a pH of 5.6. This is slightly acidic due to the presence of carbon dioxide in the atmosphere which forms weak carbonic acid in water. It is not uncommon for acidified rainwater to have a pH of 4, about 30 times as acidic as normal rainwater.
Websites
Other topics
Environment Canada
pH Scale
US Park Service
Introduction
Agreements
Buildings
Cars
Chimneys
Critical Loads
Deposition
Doing Our Bit
Emissions
Europe
Fossil Fuels
Freshwater
Impacts
Industry Controls
Industry & Power
Liming
Measuring
Modelling
Monitoring
Natural Sources
Nitrogen Oxides
Rainfall Acidity
Soils
Sulphur Dioxide
Transboundary
Trees
UK Acid Rain
Vehicle Controls
Wildlife
 Print Topic
Print Topic